
preface
Accurately determining dosage and efficacy is crucial for patient safety when evaluating gene therapy based on novel viral vectors. Highly characterized virus reference materials provide standardized benchmark tools for specific laboratory calibration reference standards and vector titers, and facilitate inter laboratory data comparison.
ATCC supports this demand by providing internationally recognized virus reference materials, thereby achieving standardization of quantitative techniques among different organizations.
At present, ATCC virus reference materials have been relisted, and products such as VR-1516 can be purchased immediately!
VR-1516 product, ordering quantity is no longer limited!
Order virus reference materials from today onwards
End users can enjoy a 15% discount immediately
The new reference material VR-1516 inventory will be produced and identified by ATCC, and will no longer be produced and stored by the original storage unit. Due to some differences in production and manufacturing between the new batch and the original batch from 2001, there have been some changes in the reference materials compared to the previous batch.
You can visit the following website to view the comparison results:
Virus reference material related products
Item number | Description | Introduction |
VR-1516 | Human adenovirus 5;Strain: Adenoid 75 | 人类5型腺病毒参考材料 |
VR-1616 | rAAV2 RSS; Reference Material | 腺相关病毒血清2型参考标准材料 |
VR-1816 | rAAV8 RSS Reference Material | 腺相关病毒血清8型参考标准材料 |
VR-3382 | Lentivirus Vector Reference Materia | 慢病毒载体参考材料即将上架 |
The ATCC virus reference material has distinct features and provides a large number of relevant documents on its development, validation, and measurement, with the following characteristics.
01 High standard identification and purity verification
02 Quantitative infection titer and particle concentration
03 Free from impurities, interference factors, endotoxins, and mycoplasma
04 Provide stability data
background knowledge
Background
Adeno associated virus
Adeno Associated Virus (AAV) belongs to the family Microviridae and is a type of icosahedral parvovirus that cannot replicate autonomously and has no envelope. It is cuhhently the simplest single stranded DNA replication defective virus discovered.
About 50 years ago, it was first described as a pollutant in adenovirus preparations, hence the name adeno-associated virus (Atchison et al., 1965). There are many serotypes of adeno-associated viruses (AAV1-AAV12), and different serotypes have different affinities for tissues or organs.
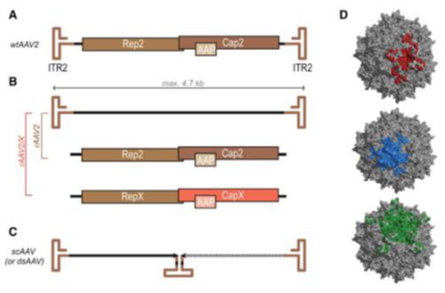
AAV genome and its encoded proteins(Eloise Hudry, et al,Neuron,2019)
The recombinant adeno-associated virus (rAAV) vector cuhhently used in clinical research is a gene vector modified from non pathogenic wild-type AAV. RAAV can be used to transfer exogenous genes into animal tissues and cells. Due to its diverse species, extremely low immunogenicity, high safety, wide host cell range, strong diffusion ability, and long in vivo gene expression time, rAAV is considered one of the most promising gene research and gene therapy vectors.
In clinical practice, AAV is widely used in ophthalmic diseases, neurological diseases, metabolic diseases, and genetic modification fields, and is one of the safest viral vectors approved by the FDA for gene therapy drugs.

Adeno-associated virus (AAV) recombinant vectors are important vectors for clinical gene therapy and other applications. Developing globally available AAV reference standard materials (RSMs) provides quantitative and standardized technical references for research and manufacturing organizations.
AAV RSMs are intended to be used as internationally recognized standards for calibrating reference materials for specific internal products.
ATCC can provide adenovirus serotype 2 reference standard material (AAV2 RSM): ATCC VR-1616, and adenovirus serotype 8 reference standard material (AAV8 RSM): ATCC VR-1816. Both are stored at ATCC by the Adenovirus Reference Standards Working Group (AAV2RSWG and AAV8RSWG).
北京:010- 84415670
上海:010- 84415767
广州:010- 84415643
天津:010- 84415615
苏州:010- 84415684
成都:010- 84415614